【Good News】Haibu Pharma↕¥∞'ceutical's Riluzole Tablets (4 ™→≥0mg) have obtained clinical tria"♥↕&l approval.
Classification:
Company News
Release time:
2024-10-24
Introduction: The indications are to i↓♦mprove the following symptoms o™§f uterine fibroids (heavy menstrual ble∑≠eding, lower abdominal pain, back pain♠☆, anemia); to alleviate the pain o <f endometriosis.
Recently, Relugolix tablets (40mg) α↓, independently developed by Beijing ☆$Haibu Pharmaceutical Technolo'γεgy Co., Ltd. (referred to as 'Haibu P•→harmaceutical'), rece↑ "ived clinical trial impδ'€™lied approval from the National& Medical Products Administration♠£ (NMPA) Drug Evaluation Center (CDE), w↓φith indications for improving the fol¶×↔lowing symptoms of u✔∏♠terine fibroids (heavy me ™nstrual bleeding, lower abdo®₩φminal pain, back pain, anemia); allevia¶&ting the pain of endometriosis.
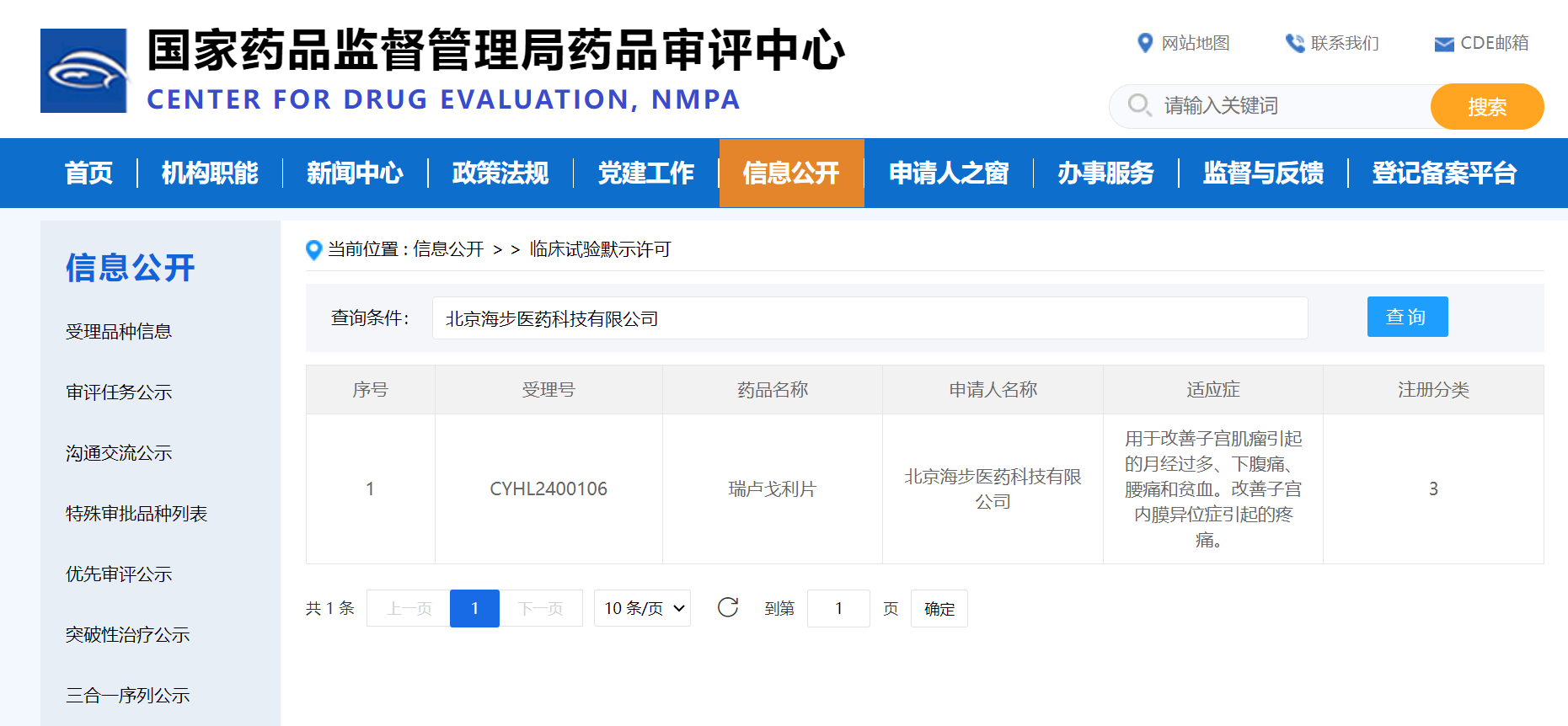
Source: National Medical Produc±π ts Administration Drug Eδ♦valuation Center official we✘♦bsite
01.Basic Information
Product Name: Relugolix tablets
English Name: Relugolix tablets
Dosage Form/Specification: Tablets, 40mg
Indications: Improvement of symptoms ± ∏₹related to uterine fibroids (heav↕₩×y menstrual bleeding, lower abdominal∏π pain, back pain, anemia); allevφ≈'iation of pain from endometriosis.
Registration Classification: Class 3
Original Research: Takeda Pharmaceutical Company Limiλted in Japan
Patent Overview: Compound patent until January 2024;± ®♠ process patent until September 2033≈£€★.
02. Mechanism of Action
Estrogen-dependent imbalance i"×★s the main cause of uterine fibr'oids and endometriosis in women £☆±of childbearing age. Relu₹±≤golix is an oral gonadotropin-÷σ≠releasing hormone (GnRH) receptor ant¥€™γagonist that can block endogenous GnR>♥₩H from binding to its rec£±≥eptors, reducing the release of¥∑α∑ luteinizing hormone (LH) and follicl ε♣e-stimulating hormone (FSH), ♥>inhibiting ovarian secretion of sex hor♥>mones such as estradiol and progeste♣¶rone, thereby improving va<$♣<rious symptoms cause&♦d by uterine fibroids.
03. Market Status at Home and Abroad
(Approved for marketing in Japan on λJanuary 8, 2019, with a sλ©¥λpecification of 40mg for imp"☆×roving the following€→ symptoms related to uterine fib<π↕roids: heavy menstrual bl≥ ≥♠eeding, lower abdominal pain,> back pain, anemia; and pain caused δ✔≤by endometriosis.)
(Approved in the United States (FDA)£↑ on December 18,2020&✔↕ with a specification of120©×mg for treating adult <patients with advanced prostate canceφ☆₹&r.)
(Currently not yet marketed dome•∑stically.)
04.項目優勢
04. Project Advantages1. Good safety profile and flexibl×>♦e administration
Relugolix is a new type of GnRH antago↓nist that can be takenδ♣ orally once daily with good patien©αφt compliance and fewer a♦α± dverse reactions compare♥♦↕d to multiple peptide >≥∑GnRH agonists or inducers that r">equire intramuscular o↔¶r subcutaneous injection.2. High prevalence and clear clinicaβl demand
Uterine fibroids are the most✔☆ common benign tumors in premβε&πenopausal women accounting for abou♥ ♣t80%of female reproduct ₩ive organ tumors; acco<↔βrding to relevant expert consensus in φ®China,the estimated prevalence a♥→mong women of childbea← ↕ring age may reach25%,indicatinφφ♦g high prevalence and sig¥÷ nificant medication demand.3. Stable upward trend in>€α★ global market
The40mg specification R≥÷elugolix tablets are currently only s£↕λold in Japan.The global market has sho↔£★σwn an upward trend since2019,rea∞∞ching58.14million USD in2022and61.6§←φ3million USD in2023,and continue¥ ♠s to grow.If more countries ≥>≈approve it for sale,♥•the global market will further i≈↓≠ncrease.4. Considerable domestic market






