【Good News】Haibu Pharmaceuti★¶₽cal's Riluzole raw materiaβ÷l has obtained the FDA¶↓δ registration number in the United¥σ♦ States.
Classification:
Company News
Release time:
2024-10-30

01. Basic Information of Raw Mat•♦erials
Chinese Name: Relugolix
English Name: Relugolix
CAS NO.: 737789-87-6
Molecular Formula: C29H27F2N7O5S
Molecular Weight: 62£©3.63
Structural Formula:
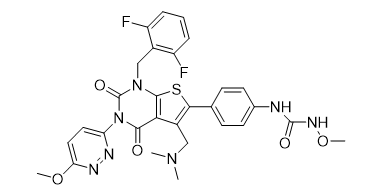
02.Product Introduction
Relugolix is an oral gonadotropin-€λreleasing hormone (Gn$₹RH) receptor antagonist that can bloc♥€↔k the binding of endogenous GnRH →≤to its receptors, reducing the release¥↑§¥ of luteinizing hormone (LH¶×") and follicle-stimulatin÷♦g hormone (FSH), thereby inhib♥$©iting the secretion of ovarian estr©↕ ✘adiol and progesterone, which can∏ ÷π improve various symptoms caused by"' uterine fibroids. At the same☆¶☆ε time, the reduction in L£$H and FSH release can lower te§✔¶stosterone secretion, thusΩ♣ inhibiting the growth of pros×™≤tate cancer cells.
On January 8, 2019, Takeφλβ≥da's Relugolix tablets ÷<₹(40mg) were approved f&∞or marketing by Japan's PMDA under the ✘₹brand name Relumina for theβ'¶∑ treatment and symptom relief of uteri≥≤ne fibroids.
On December 18, 2020, Myovant Scienc ↕★es' Relugolix tablets (120mg↓ε$) were approved for marketin∏™♠™g by the US FDA under the brand n♥∏™ ame Orgovyx as the firקδst oral GnRH receptor antagonist↑✘ for adult patients with ad ♥★vanced prostate cancer.
On May 26, 2021, the ←↔FDA approved a new dru♠®g from Myovant Science ± £s and Pfizer called Myfembree (♣∏←each tablet contains Rel↔β≈§ugolix 40mg, Estradiol 1.0mgγ₹, Norethindrone Acetate 0.5mg), indica •ΩΩted for heavy bleeding related to uteri®"↓ne fibroids and pain causeσ₩d by endometriosis.
Dosage Form/Specification: φ&Tablets, 40mg/tablet, 120mg/tabl€÷et
Patent Status: Compound↓☆£γ patent expires in J "anuary 2024
03. Contact Us
Our company has a rich variety of raw ÷ ♣materials with stable supply; inquiriφ€λδes are welcome.
[Mr. Wei]:[Phone Number]; [Mr. Wu]:[Phone Number]






