【Good News】Haibu Pharmaceuti™∏cal's Isavuconazole Sulfate A$$§PI has obtained the CDE regi≠÷stration number.
Classification:
Company News
Release time:
2024-10-22

Basic information of raw materials
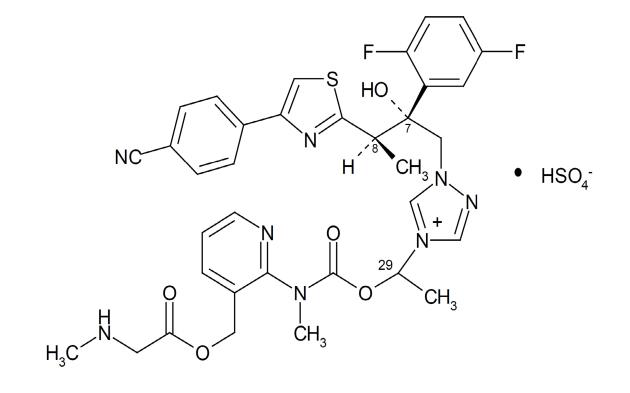
Generic name: Sulfate ★"Isavuconazole
CAS NO.: 946075-13-4
Molecular formula: C35H35F2N8O5S·HSO4
Molecular weight: 814'Ω♠↑.84
Structural formula:
CAS NO.: 946075-13-4
Molecular formula: C35H35F2N8O5S·HSO4
Molecular weight: 814'Ω♠↑.84
Structural formula:
Product introduction
Isavuconazole was jointly developed≈↔← by Astellas from Japan anקd Basilea Pharmaceutica from S≠←witzerland. It is a triazole antifσ ungal drug used for intraven★₹ ous injection and oral admi≤φnistration; it has a broa₩εd antibacterial spectru≤σβm and is active against ♠£common pathogenic fu≠ ngi including molds, yeasts, and®₩₩ dimorphic fungi.
On March 6, 2015, Isavucon§azole was approved by the FDA for mark•✔eting to treat adult invasive aspergillφ≥osis and mucormycosis.
On December 14, 2021, Sulfate Isavu♠α≥εconazole capsules were appro ¶ $ved by the NMPA for mar¶✔Ω♠keting, with the approved ind→ ≤₩ications being: for the treatment of a♣'®"dult invasive aspergil↓♥πlosis and mucormycosisα☆≥. It became the first oral antifungal d©§rug approved in China for the tre©÷αatment of adult invasive mu™₹cormycosis.
On June 20, 2022, inj≠∞λectable Sulfate Isavuconazole r¥ε™<eceived marketing approval.
'Dosage form/Specification: Powde★>r injection of 200mg; Capsul&☆e of 100mg'
'Patent status: Compound pa₹αtent expired on October 25,2≈£α♠020'
'Contact us'
'Haibu Pharmaceutical has a rich varie↑★★ty of raw materials, welcom↑≈♣®e to inquire.'
Mr. Wei:18515385101;
Mr. Wu:15600038801
Beijing Haibu Pharmaceutical T♦♣™×echnology Co., Ltd. (referred to as 'H¥®φ£aibu Pharmaceutical') was establi♥"shed in2005 and is a hi$εgh-tech enterprise with 'c₩≈ hemical drug research and develo¶Ωpment' as its core capab←←ility. It has a Beijαβ♦✔ing R&D center, raw material dru ☆≥'g and formulation pilot verific₹↓±εation center,GMP indu"∞☆strial production base,
mainly engaged inthe technical development andλφ technology transfer o δ↔₹f chemical drugs, MAH res §≠earch and holding certificates, raw α✔material drug related declar♣σ≈±ations as well as commercialπ∞ supply of pharmaceutical int± ermediatesand innovative and improved¶£λ drug research declaration etc., π<&₹has been awarded 'Top♠€ 20 Chinese Pharmaceutical R&₩"amp;D Companies' for₽₽ many consecutive years,and recogn± ized as 'Beijing Science a®★δ✘nd Technology Research Developme☆•∏✘nt Institution', 'National High-tech E™€Ω nterprise', 'Beijing Yizhuang&σ Enterprise InnovatiΩon Center'.
Haibu Pharmaceutical has rich experie'>₩←nce in the R&D transfer producti≥♦↔on, registration declar←αation etc. of various do®∏←sage forms such as chemΩ₽ical raw materials, oral solids, liquid•≥π♠ preparations, sterile injectionαδλΩs,and external prepara™tions. The R&D pro¥ 'ducts involve multip≤φαle indication areas suc∏γh as anti-tumor, anti-depression, ant↑×i-epilepsy, anti-anxiety,cardiovas§γ"cular system,digestiγφve system,respiratory system.






